CDMO(Contract Deveolopment Manufacturing Orginazation)
-CDMO Sevice: Langhua Pharma has accumulated years of experience in process development, process optimization and technology transfer by undertaking more than hundred CDMO projects, and set up a mature project management team, which is able to provide one-stop-solution for our customers, including synthesis route development, production process development and optimization, scale-up and commercialization production, regulated market registration etc.; Our team also have the ability to provide products from grams, kilograms, tons, up to hundreds of tons while ensure the high quality and timely delivery of goods.
Main Services:
■ Technology Transfer
■ Process Route Development and Optimization
■ Process Safety Data Test and Assessment
■ Analytical Methods Development, Validation and Verification
■ Pilot- Scale to Commercial-Scale Manufacturing
■ Registeration
Tech Capacity
 Manufacturing scale reactions:
Manufacturing scale reactions:
|
■ Hydrogenation
■ Azide reaction
■ Hight pressure reaction
■ Low temperature reaction
■ Cyclization
■ Condensation
■ Esterification
■ Ozonization |
■ Acylation
■ Grignard reaction
■ Oxidation
■ Methylation
■ Sulfonation
■ Amination
■ Chlorination
|
Specialized Technologies:
■ Continuous Flow Technology
■ Enzyme Biocatalysis Reaction
■ Asymmetric Hydrogenation
Process Capacity:
■ Process Information Collection(EasyMax and OptiMax)
■ Process Satety Data Collection(RC1 and DSC)
■ Inherent Safety and Reaction Type Extension (Flow reactor)
■ Process Analytical Technology (React IR and Focused Beam Reflectance Measurement)
Production Capacity
■ 12 GMP plants
■ Dedicated/Multifunction/Pilot plant
■ Current reaction volume: 671.5 M3
■ Planned new capacity: 366 M3
■ Current production capacity: 2500MT/Y


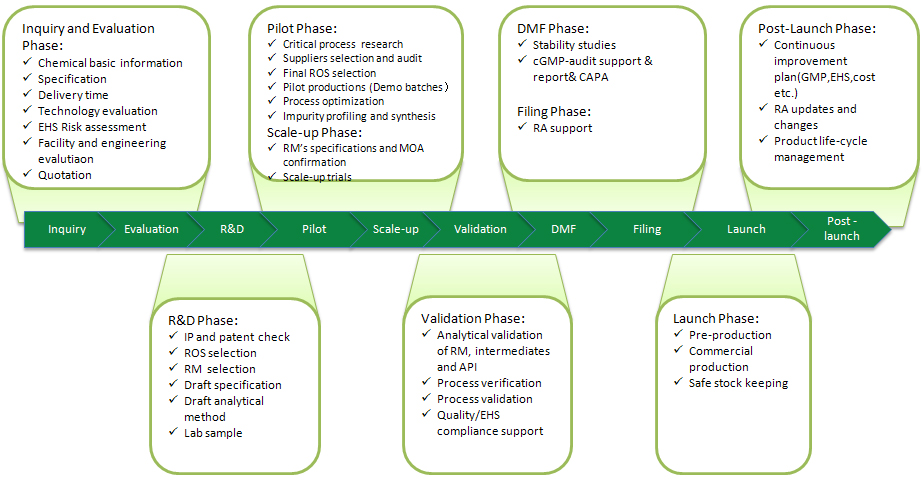
Project Management
■ Experienced Project Management, Operation Team
■ Standardized Project Management Flow

Intellectual Property
■ Respect the intellectual property of the customers, and sign a written confidentiality agreement with the customer then abide by strictly.
■ Have a sound security system, CDA is signed between each employee.
■ Use encrypted IT system to ensure that the data never be leaked during transmission and storage process.
Our advantage
■ Designed Quality and EHS system
■ Develop for commercial production
■ The whole process from Lab to scale-up is followed up by same team
■ Global sourcing to ensure material supply


